Last Updated on October 29, 2023
The adult human skeleton has a total of 206 bones, excluding the sesamoid bones. The appendicular skeleton has 126 bones, axial skeleton 74 bones, and auditory ossicles six bones.
[Read more about different bones and their classification]
Each bone possesses a unique shape. But the bones across the body do possess similarities in terms of structure and physiology. Bones can be grouped according to these similarities.
A common physiologic thread across all the bones is constant modeling and remodeling throughout the life to help them adapt to changing biomechanical forces and to remove old, microdamaged bone and replace it with new, mechanically stronger bone. Constant removal and renewal help to preserve the bone strength.
The four general categories of bones are long bones, short bones, flat bones, and irregular bones.
- Long bones include the clavicles, humeri, radii, ulnae, metacarpals, femurs, tibiae, fibulae, metatarsals, and phalanges.
- Short bones include the carpal and tarsal bones, patellae, and sesamoid bones.
- Flat bones include the skull, mandible, scapulae, sternum, and ribs.
- Irregular bones include the vertebrae, sacrum, coccyx, and hyoid bone.
Flat bones form by membranous bone formation, whereas long bones are formed by a combination of endochondral and membranous bone formation.
Parts of Bone
Before ossification is complete the following parts of the bone can be defined. [After ossification, physis fuses with rest of bone and bone has a shaft, metaphysis, and articular ends.
Each long bone has an elongated shaft or diaphysis and two expanded ends (epiphyses) which are smooth and articular. The shaft typically has 3 surfaces separated by 3 borders, a central medullary cavity, and a nutrient foramen directed away from the growing end. Limb bones are typical long bones. Examples are humerus, radius, ulna, femur, tibia and fibula, metacarpals, metatarsals, and phalanges.
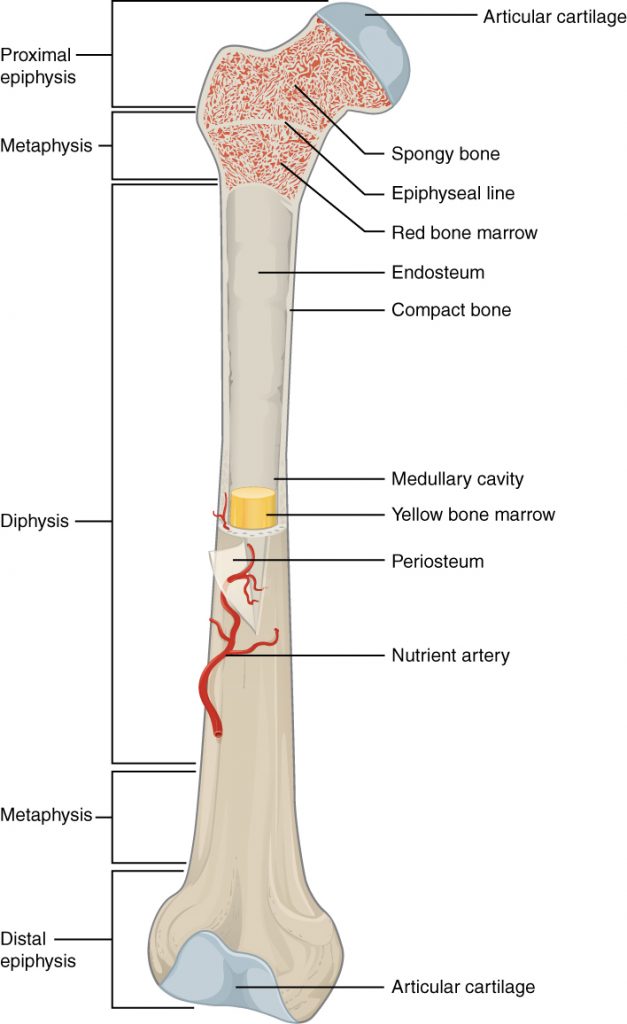
Epiphysis
The end and tips of a bone which ossify from secondary centers are called epiphyses. These are of the following types:
Pressure epiphyses
This is articular and takes part in the transmission of the weight. Examples: head of the femur, the lower end of radius, etc
Traction epiphysis
It is nonarticular and does not take part in the transmission of the weight. It always provides attachments to one or more tendons which exert a traction on the epiphysis. The traction epiphyses ossify later than the pressure epiphyses. Examples: trochanters of femur and tubercles of the humerus.
Atavistic epiphysis
It is phylogenetically an independent bone which in man becomes fused to another bone. Examples: coracoid process of scapula and os trigonum.
Diaphysis
It is the elongated shaft of the bone which ossifies from a primary center.
Metaphysis
The ends of the shaft towards physis are called metaphyses. Each metaphysis is the zone of active growth. Before epiphyseal fusion, the metaphysis is richly supplied with blood through end arteries forming ‘hair-pin’ bends.
This is the common site of osteomyelitis in children because, the bacteria or emboli are easily trapped in the hair-pin bends, causing infarction. After the epiphyseal fusion, vascular communications are established between the metaphyseal and epiphyseal arteries.
Epiphyseal Plate of Cartilage
It separates epiphysis from metaphysis. The proliferation of cells in this cartilaginous plate is responsible for the lengthwise growth of a long bone. After the epiphyseal fusion, the bone can no longer grow in length.
The growth cartilage is nourished by both the epiphyseal and metaphyseal arteries.
Sections of Bone
Naked eye examination of the longitudinal and transverse sections of a bone shows the following features:
Shaft
From without inwards, it is composed of periosteum, cortex and medullary cavity.
Periosteum
Periosteum is a thick fibrous membrane covering the surface of the bone. It is made up of an outer fibrous layer, and an inner cellular layer which is osteogenic in nature. Periosteum is united to the underlying bone by Sharpey’s fibers, and the union is particularly strong over the attachments of tendons and ligaments.
At the articular margin, the periosteum is continuous with the capsule of the joint. The abundant periosteal arteries nourish the outer part of the underlying cortex also. Periosteum has a rich nerve supply which makes it the most sensitive part of the bone.
Cortex
Cortex is made up of a compact bone which gives it the desired strength to withstand all possible mechanical stains.
Medullary Cavity
Medullary cavity is filled with red or yellow bone marrow. At birth, the marrow is red everywhere with widespread active hemopoiesis.
As the age advances the red marrow at many places atrophies and is replaced by yellow, fatty marrow, with no power of hemopoiesis.
Red marrow persists in the cancellous ends of long bones. In the sternum ribs, vertebrae and skull bones the red marrow is found throughout life.
Ends of Bone
In adults, the cut section would show cancellous bone with articular margins.
[Know about other types of bones in the body]
Development of Long Bone
In early fetal life, the bone is just a framework formed by the cartilage. The body needs to convert this into the bony material. This is achieved by ossification centers, the nidus from where bone tissue is constructed and laid over the cartilage framework.
Typically, three ossification centers are responsible for ossification of long bone cartilage framework.
One is in the shaft which appears early and two are at the ends of the bone which appear late. Special cells called osteoblasts lay down mineralized channels into the cartilage.
Ultimately, all the cartilage is resorbed and replaced by bone tissue except for two transverse thin plates of cartilage that remain for future growth of the bone.
This cartilage tissue is called physis and responsible for the growth of the bone. One of the physis is responsible for the greater growth of length than other.
Just behind the physis [towards the center of the bone] are widened areas of bone called metaphysis. Metaphyses has cancellous bone as compared to the cortical bone of the shaft [which is the length of the bone between two metaphyses]. As the bone grows and adds more bone, the part of the metaphysis farther from physis [towards the center of the bone] is remodeled and incorporated into the shaft as cortical bone.
The shaft is called diaphysis.
The epiphysis is the segment of the bone between physis and end of the bone.
Thus, two epiphyses, two physes, two metaphyses, and one shaft constitutes a long bone.
Thus a typical long bone ossifies into three parts, the two ends from secondary centers, and the intervening shaft from a primary center.
Functions of Bones
The skeleton serves a variety of functions.
- Bones give shape and support to the body and resist all forms of stress
- They provide a surface for the attachment of muscles, tendons, ligaments etc.
- They serve as levers for muscular actions
- The skull, vertebral column, and thoracic cage protect the brain, spinal cord, and thoracic viscera, respectively
- Bone marrow manufactures blood cells
- Bones store 97% of the body calcium and phosphorus
- Bone marrow contains reticuloendothelial cells which are phagocytic in nature and take part in the immune response of the body.
Composition and Structure of Bone
Broadly speaking, the bone consists mainly of collagen fibers and an inorganic bone mineral in the form of small crystals. In the living body, 10-20% of bone mass is made of water. Minerals form approximately 60-70% of the dry bone mass and rest is collagen, a small amount of some other proteins and inorganic salts.
Calcium and phosphorus are major bone minerals and form crystals in form of hydroxyapatite [Ca10(PO4)6(OH)2]. Bone has calcium phosphorous ration in varying proportion of 1.37 – 1.87. Additional ions such as silicon, carbonate, and zinc are also present.
Bone mass accounts for 50 to 70% of bone strength. For this, both geometry and composition are important.
As bone diameter expands radially, the strength of bone increases by the radius of the involved bone raised to the fourth power. That is why larger bones are stronger than smaller bones, even with equivalent bone mineral density.
The amount and proportion of trabecular and cortical bone at a given skeletal site affect bone strength independently.
Conditions like osteomalacia, fluoride therapy, or hypermineralization states affect the mineral component and microstructure, thus affecting the bone strength.
Cortical and Cancellous Bone
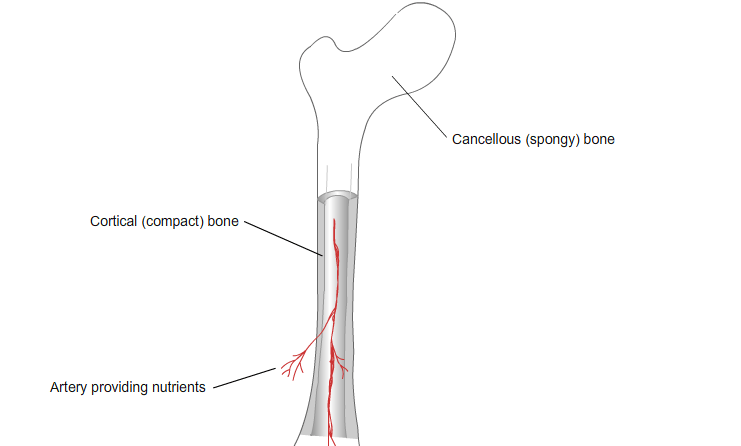
Each bone has a unique shape according to its location and function. Broadly bones can be classified into two types according to their structures.
- Cortical or Compact
- Cancellous or Spongy
Eighty percent of the skeleton is composed of cortical bones whereas 20% is cancellous in human adults. Different bones may have different ratios of cortical to trabecular bone.
For example, the vertebra is composed of cortical to cancellous bone in a ratio of 1:3 where has femoral head has a ratio of 50:50.
95% of diaphyseal bone is cortical.
Cortical Bone
Cortical bone is the denser, stronger of the two types of and found majorly in the diaphyses of long bones, where it provides support and protection.
Osteon is the basic building block or microscopic structural unit of cortical or cancellous bone. Osteons of cortical bone are called Haversian system whereas osteons of cancellous bone are called packets. Haversian systems are cylindrical in shape, are approximately 400 mm long and 200 mm wide at their base
Each osteon is composed of concentric rings of calcified matrix called lamellae. The centermost concentric lamella surrounds a Haversian canal and layers of lamellae are arranged around it circumferentially. Haversian canal contains a vein, artery, nerve, and lymph vessel.
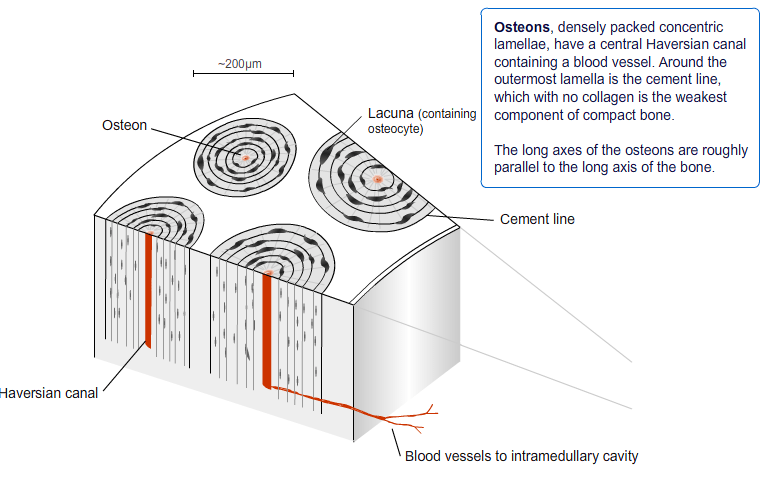
These vessels and nerves branch off at right angles through a perforating canal, also known as Volkmann’s canals, to extend to the periosteum and endosteum. Thus Volkmann’s canals are in a direction perpendicular to Haversian canal.
A set of concentric lamellae, its associated lacunae and the vessels and nerves of the central canal are collectively called an osteon.
This arrangement is continued the whole length of cortical bone as series of concentric tubes. Multiple osteons are stacked together with interspersed collagen and cement at the junction of lamellae and collagen.
The open areas between neighboring osteons are also filled with compact bone. This “filler” bone is referred to as the interstitial lamellae.
Cortical bone has an outer periosteal surface and inner endosteal surface. Periosteal surface activity is important for appositional growth and fracture repair.
During the growth phase, bone formation exceeds bone resorption on the periosteal surface. This leads to increase in diameter/girth of the bone with aging.
At the same time, bone resorption exceeds bone formation on the endosteal surface leading to expansion of marrow space.
There are small cavities between the layers of concentric lamellae called lacunae. Osteocytes reside in lacunae at the borders of adjacent lamellae.
Bone canaliculi are microscopic canals between the lacunae. The radiating processes of the osteocytes (called filopodia) project into these canals. These cytoplasmic processes are joined together by gap junctions. The space not occupied by osteocytes is called periosteocytic space and is filled with periosteocytic fluid which contains substances too large to be transported through the gap junctions.
If you look further into the osteon, the collagen fibers are arranged in alternating directions.
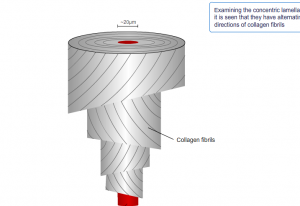
Further microscopy shows hydroxyapatite crystals adhered to collagen fibrils.
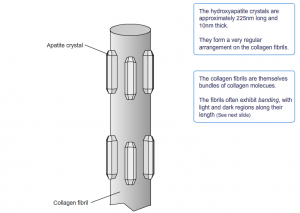
These also incorporate other inorganic salts like magnesium hydroxide, fluoride, and sulfate as it crystallizes, or calcifies, on the collagen fibers. The hydroxyapatite crystals give bones their hardness and strength, while the collagen fibers give them flexibility.
Cancellous or Spongy Bone
Unlike cortical bone, osteons of cancellous bone, called packets are not arranged in concentric circles. Instead, the lacunae and osteocytes are found in a lattice-like network of matrix spikes called trabeculae. Trabecular plates and rods, 50 to 400 mm in thickness form honeycomb arrangement interspersed in the bone marrow compartment. Trabecular osteons are semilunar in shape, normally approximately 35 mm thick, and composed of concentric lamellae.

The trabeculae may appear to be a random network, but each trabecula forms along lines of stress to provide strength to the bone. The spaces of the trabeculated network provide balance to the dense and heavy compact bone by making bones lighter so that muscles can move them more easily. In addition, the spaces in some spongy bones contain red marrow, protected by the trabeculae, where hematopoiesis occurs.
Lamellar Bone and Woven Bone
Both cortical and cancellous bones are formed by lamellar pattern, in which collagen fibrils are laid down in alternating orientations.
Therefore, these two bones are also called lamellar bones. It is important to differentiate lamellar bone from woven bone as the lamellar bone has significant strength as a result of the alternating orientations of collagen fibrils.
This lamellar pattern is absent in woven bone, in which the collagen fibrils are laid down in a disorganized manner. Woven bone is weaker than lamellar bone. Woven bone is normally produced during formation of primary bone, fracture healing and also in hyperparathyroidism.
Periosteum and Endosteum
Bone is surrounded by a layer of fibrous connective tissue. This is called periosteum. Exceptions are articular surfaces where the bone is covered by cartilage instead.
The periosteum contains blood vessels, nerve fibers, and osteoblasts and osteoclasts. The periosteum is tightly attached to the outer surface of the bone by thick collagenous fibers. These are called Sharpey’s’ fibers. These extend into underlying bone tissue. [search and write]
The endosteum is a membranous structure covering the inner surface of the bone and extends to Volkman’s canals. The endosteum is in contact with the bone marrow space, trabecular bone, and blood vessel canals and contains blood vessels, osteoblasts, and osteoclasts.
Blood Supply of Bones
The blood of a long bone is derived from the following sources –
Nutrient Artery
Nutrient artery enters the shaft through the nutrient foramen, runs obliquely through the cortex, and divides into ascending and descending branches in the medullary cavity.
Each branch divides into a number of small parallel channels which terminate in the adult metaphysis by anastomosing with the epiphyseal, metaphyseal and periosteal arteries.
The nutrient artery supplies the medullary cavity, inner two-thirds of cortex and metaphysis.
The nutrient foramen is directed away from the growing end of the bone.
Periosteal arteries are especially numerous beneath the muscular and ligamentous attachments. They ramify beneath the periosteum and enter the Volkmann’s canals to supply the outer one-third of the cortex.
Epiphyseal Arteries
Epiphyseal vessels are derived from periarticular vascular anastomosis and enter through vascular foramina.
The number and size of these foramina may give an idea of the relative vascularity of the two ends of a bone.
Metaphyseal arteries
These are derived from the systemic vessels in the vicinity of the bone. They pass directly into the metaphysis and reinforce the metaphyseal branches from the primary nutrient artery.
Lymphatics have not been demonstrated within the bone, although some of them do accompany the periosteal blood vessels, which drain to the regional lymph nodes.
Bone Cells
Although bone cells compose a small amount of the bone volume, they are crucial to the function of bones. Four types of cells are found within bone tissue
Osteogenic Cells
Osteogenic cells are undifferentiated osteoprogenitor cell which would develop into osteoblasts. These cells have high mitotic activity.
Immature osteogenic cells are found in the deep layers of the periosteum and the marrow. They differentiate and develop into osteoblasts.
Osteoblasts
Osteoblasts are the cells derived from osteoprogenitor cells or osteogenic cells. These are responsible for forming new bone and is found in the growing portions of bone, including the periosteum and endosteum.
Those osteoblasts, which do not divide, synthesize and secrete the collagen matrix and calcium salts.
Osteoblasts synthesize new bone matrix [collagen and other matrix proteins] on bone-forming surfaces and from osteocytes within the bone matrix to support bone structure. They also have protective lining cells that cover the surface of the endosteal surfaces. They are also present under the periosteum.
Osteoblasts are heterogeneous which might explain different trabecular microarchitecture at different bone sites, the difference in diseases disease states at different anatomical sites and different responses of different regions to respond to agents for bone diseases.
Osteocytes
As the secreted matrix surrounding the osteoblast calcifies, it traps the osteoblast, which changes shape and becomes osteocyte.
The osteocyte is the primary cell of mature bone. It is also the most common type of bone cell. Each osteocyte is located in a space called a lacuna and is surrounded by bone tissue.
Osteocytes are responsible for the mineral concentration of the matrix by secreting enzymes. Osteocytes do not normally express alkaline phosphatase but do express osteocalcin, galectin 3, and CD44, a cell adhesion receptor for hyaluronate, as well as several other bone matrix proteins.
Osteocytes communicate with each other and receive nutrients via long cytoplasmic processes or filopodia that extend through canaliculi, channels within the bone matrix. They regulate the exchange of mineral in the bone fluid within lacunae and the canalicular network
Osteocytes are active during osteolysis and may function as phagocytic cells.
Osteocytes also take part in mechanosensation. They transduce stress signals from bending or stretching of bone into biologic activity.
Osteoclasts
Osteoclasts are cells that resorb bone. Osteoclasts are derived from mononuclear precursor cells of the monocyte-macrophage lineage.
RANKL and macrophage colony stimulating factor are two cytokines that are critical for osteoclast formation. Latter is also required for the proliferation, survival, and differentiation of osteoclast precursors, as well as osteoclast survival and cytoskeletal rearrangement.
Bone resorption depends on osteoclast secretion of hydrogen ions and cathepsin K enzyme.
Bone Extracellular Matrix
Bone protein is composed of 85 to 90% collagenous proteins, mainly type I collagen, with trace amounts of types III, V, IX, XII, XIV, XIX, XX, and XXI. Noncollagenous proteins compose 10 to 15% of total bone protein.
Approximately 25% of noncollagenous protein is derived exogenously, including serum albumin and glycoprotein. These proteins help in the regulation of matrix mineralization and bone cell proliferation.
Other exogenously derived noncollagenous proteins are growth factors and molecules in that may affect bone cell activity.
Osteoblasts also synthesize and secrete noncollagenous proteins The various proteins are grouped into proteoglycans, glycosylated proteins and carboxylated (gla) proteins.
Osteocalcin is regarded as a marker of bone turnover.
Alkaline phosphatase is a glycosylated protein that plays an undefined role in mineralization of bone.
The most prevalent noncollagenous protein in bone is osteonectin [2%] and is thought to affect osteoblast growth and matrix mineralization.
Mineralization of Bone Matrix
Mineral constitutes about 50 to 70% of bone [Organic matrix is 20-40%, 5-10% is water and about 3% is fat.
The mineral content of bone is mostly hydroxyapatite [Ca10(PO4)6(OH)2] crystals which measure about 200 Å, with small amounts of carbonate, magnesium, and acid phosphate.
Matrix maturation is associated with expression of alkaline phosphatase and several noncollagenous proteins, including osteocalcin, osteopontin, and bone sialoprotein. These calcium- and phosphate-binding proteins are thought to help the regulation of ordered deposition of mineral by regulating the amount and size of hydroxyapatite crystals formed.
Bone mineral is initially deposited in “hole” zones between the ends of collagen fibrils. This process may be facilitated by extracellular matrix vesicles in bone.
Confirmed mineralization promoters or nucleators include dentin matrix protein 1 and bone sialoprotein. Bone alkaline phosphatase may increase local phosphorus concentrations, remove phosphate-containing inhibitors of hydroxyapatite crystal growth, or modify phosphoproteins to control their ability to act as nucleators.
Vitamin D plays indirectly stimulates mineralization of unmineralized bone matrix. It also promotes differentiation of osteoblasts and stimulates osteoblast expression of bone-specific alkaline phosphatase, osteocalcin, osteonectin, and a variety of other cytokines. It also influences the proliferation and apoptosis of other skeletal cells, including hypertrophic chondrocytes.
Bone Growth, Modeling, and Remodeling
Bone undergoes two kinds of growths – longitudinal and radial growth. These occur during the growing years.
Longitudinal growth is responsible for the increase in the length of the bone [and contributes to the growth of skeleton] whereas radial growth leads to an increase in girth of the bone. Longitudinal growth occurs at the growth plates, where cartilage proliferates in the epiphyseal and metaphyseal areas of long bones, before subsequently undergoing mineralization to form primary new bone.
Apart from these growths, bone is constantly subjected to modeling, and remodeling during life.
Modeling is the process by which bones change their overall shape in response to physiologic factors or mechanical forces, leading to gradual adjustment of the skeleton.
This may include widening of the bones or change in axes of the bones. This is achieved by removal or addition of bone to the appropriate surfaces by independent action of osteoblasts and osteoclasts in response to biomechanical forces.
With aging, the bones widen by periosteal apposition of new bone and endosteal resorption of old bone.
[Bone modeling is less frequent than remodeling in adults. However, certain diseases like hyperparathyroidism, renal osteodystrophy may cause an increase in the bone modeling.]
Apart from modeling, which is in response to stresses applied, bones also undergo constant renewal and repair by a process called bone remodeling. This helps to maintain bone strength and maintain mineral homeostasis.
Remodeling involves continuous removal of osteons of old bone and replacement with newly formed matrix followed by mineralization of the matrix to form new bone.
Remodeling is very important for preventing accumulation of bone microdamage. This prevents the bone from fatigue. Bone remodeling is maintained by tightly controlled osteoclasts and osteoblasts leading to resorption of old bone and formation of new bone.
Low bone turnover leads to accumulation of microfractures. High bone turnover, with bone resorption greater than bone formation, is the main cause of microarchitectural deterioration.
Cortical bone turnover is at a rate of 2 to 3%/ whereas trabecular bone turnover is higher. Thus the cancellous bone turnover is more important for mineral metabolism.
Increased demand for calcium or phosphorus may require increased bone remodeling units or in some cases increase number of osteoclasts.
The sites for bone remodeling may be random or it may occur the areas needing repair.
Bone remodeling is increased in perimenopausal and early postmenopausal women. With aging, it slows down albeit continue at a rate higher than in premenopausal women. Bone remodeling is thought to increase mildly in aging men.
The remodeling cycle is composed of four sequential phases –
- Activation and Resorption
- Reversal and Formation.
Activation and Resorption
Activation
- Recruitment and activation of mononuclear monocyte-macrophage osteoclast precursors from the circulation
- The lifting of the endosteum [contains the lining cells] off the bone surface
- Fusion of multiple mononuclear cells to form multinucleated preosteoclasts.
- Preosteoclasts bind to the bone matrix to form annular sealing zones around bone-resorbing compartments beneath multinucleated osteoclasts.
Resorption [2-4 weeks]
- Mediated by osteoclast
- Osteoclast formation, activation, and resorption are regulated by
- RANKL or Receptor activator of nuclear factor kappa-B ligand to osteoprotegerin ratio [RANKL stimulate osteoclast recruitment and activation. Osteoprotegerin prevents RANKL function]
- IL-1
- IL-6
- Colony-stimulating factor [Stimulates osteoclast recruitment]
- Parathyroid hormone
- 1,25-dihydroxy vitamin D
- Calcitonin
- Resorbing osteoclasts secrete
- Hydrogen ions to lower the pH
- Tartrate-resistant acid phosphatase, cathepsin K, matrix metalloproteinase 9, and gelatinase from cytoplasmic lysosomes to digest the organic matrix
- Saucer-shaped lacunae called Howship’s lacunae formed on the surface of the trabecular bone) and Haversian canals in cortical bone.
- Multinucleated osteoclasts undergo apoptosis and resorption is halted
Reversal and Formation [4-6 months]
During the reversal phase, bone resorption transitions to bone formation. The signals that end of bone resorption and begin are unknown but following are thought to be involved
Reversal
- Bone matrix—derived factors such as TGF-beta, IGF-1, IGF-2 [TGF-beta decreases osteoclast resorption by inhibiting RANKL production by osteoblasts]
- Bone morphogenetic proteins
- Platelet-derived growth factor
- Fibroblast growth factor
- Strain gradient in due to cutting by osteoclasts may lead to sequential activation of osteoclasts and osteoblasts, with osteoclasts activated by reduced strain and osteoblasts by increased strain.
Bone formation
- Osteoblasts synthesize new collagenous organic matrix and regulate mineralization of the matrix by releasing small, membrane-bound matrix vesicles that concentrate calcium and phosphate.
- Osteoblasts also destroy mineralization inhibitors such as pyrophosphate or proteoglycans.
- Osteoblasts become buried in the matrix and become osteocytes. The osteocyte network within bone serves as a functional syncytium.[ A syncytium or symplasm is a multinucleated cell that can result from multiple cell fusions of uninuclear cells]. Approximately 50 to 70% of osteoblasts undergo apoptosis and the rest become osteocytes or bone-lining cells.
- Bone-lining cells may regulate influx and efflux of mineral ions into and out of bone extracellular fluid, thereby serving as a blood-bone barrier, but retain the ability to redifferentiate into osteoblasts upon exposure to parathyroid hormone or mechanical forces.
Thus osteoblasts synthesize proteinaceous matrix, composed mostly of type I collagen, to fill in resorption pits. The proteinaceous matrix is gradually mineralized to form new bone.
The end result of each bone remodeling cycle is the production of a new osteon.
The remodeling process is essentially the same in cortical and trabecular bone.
Bone Balance
Bone balance is the difference between the old bone resorbed and new bone formed. Periosteal bone balance is mildly positive, whereas endosteal and trabecular bone balances are mildly negative, leading to cortical and trabecular thinning with aging. These relative changes occur with endosteal resorption outstripping periosteal formation.
Bone is a very tough structure. The primary objective of the skeleton is to support the body load. Every day the bones undergo repeated loading as we perform our daily chores.
Bone is a very strong material which can withstand extremely high loads and by virtue of its constant repair, it maintains its strength without undergoing fatigue.
Stress and strain are two important terms in the biomechanics of the materials and in orthopedics, are important from the aspect of bone strength and fracture mechanics.
We understand them in the following short notes.
Strain
Strain is defined as the percentage of change in length of the material in relation to original length. When a force is applied to any material, such as bone, it undergoes deformation. The amount of deformation in the material relative to its original length is the strain.
When a material is pushed together, the material shortens (compressive strain), when pulled, it gets longer (tensile strain).
In terms of mathematical units, the strain can be expressed as a percentage.
Strain = (change in length/original length) x100
When a muscle contracts, the tendon can strain as much as 5% in tension during intense activities. Compressive strains in bone during peak activities only rise to about 0.3%.
The bone begins to fail at 0.7% strain or 7000 microstrain.
Stress
Stress is related to force applied and is defined as force per unit area. Newtons per square meter or Pascals is the measurement unit of stress.
Thus the stress is dependent on two factors
– The force that is applied to bone
– The area on which the stress is applied
If the same force is applied to a smaller area, it would result in greater stress than if it is applied to a widespread area.
References
- Musculoskeletal system. In: Gray’s Anatomy, 39th Ed., edited by Standring S, New York, Elsevier,2004. , pp83 –135
- Burr DB: Targeted and nontargeted remodeling. Bone 30 :2 –4,2002
- Blair HC, Athanasou NA: Recent advances in osteoclast biology and pathological bone resorption. Histol Histopathol 19: 189 –199,2004
- Silver IA, Murrills RJ, Etherington DJ: Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res 175: 266 –276,1988
- Eriksen EF: Normal and pathological remodeling of human trabecular bone: Three-dimensional reconstruction of the remodeling sequence in normals and metabolic bone disease. Endocr Rev 7 :379 –408,1986
- Bonewald L, Mundy GR: Role of transforming growth factor-beta in bone remodeling. Clin Orthop Rel Res 2S: 35 –40,1990
- Locklin RM, Oreffo RO, Triffitt JT: Effects of TGF-beta and bFGF on the differentiation of human bone marrow stromal fibroblasts. Cell Biol Int 23: 185 –194,1999
- Martin TJ, Sims NA: Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med 11: 76 –81,2005
- Landis WJ: The strength of a calcified tissue depends in part on the molecular structure and organization of its constituent mineral crystals in their organic matrix. Bone 16 :533 –544,1995
- Xing L, Boyce BF: Regulation of apoptosis in osteoclasts and osteoblastic cells. Biochem Biophys Res Commun 328: 709 –720,2005
- Clarke, Bart. Normal Bone Anatomy and Physiology. Clin J Am Society Nephro.Suppl 3 (2008): S131–S139. PMC. Web. Accessed 16 Feb. 2016.