Last Updated on October 29, 2023
Motion preservation surgery in the spine refers to a group of surgeries which aim at preservation of the spine motion by replicating normal or near normal biomechanics. The fundamental to all these surgeries is the use of devices, that replicate these functions.
Decompression and fusion are classic procedures of spine surgery. Motion preservation surgeries are relatively newer concept. The intent of motion preservation surgeries is to provide a better treatment alternative that preserves the function of the spine well.
Motion preservation surgery of the spine is a complex, constantly evolving field and the research is ongoing.
Motion Preservation Surgeries for Cervical Spine
Cervical motion preservation surgery mainly consists of total disc replacement.
Cervical Total Disc Replacement
In cervical total disc replacement, there is the removal of the diseased disc and implantation of the arthroplasty device. The goal is neural decompression, restoration of near normal motion so as to minimize adjacent level disease.
Two devices for commercial use are the ProDisc-C (Synthes Spine USA) and Prestige ST (Medtronic, Memphis, TN).
Clinical trials are underway to determine clinical effectiveness versus anterior cervical compression and fusion
Complications of cervical total disc replacement include fracture, subsidence, migration of the implant, heterotopic ossification, and hardware failure or loosening. Subsidence, as in lumbar TDR, is a gradual process whereby the device settles into the adjacent vertebral body.
Motion Preservation Surgeries for Lumbar Spine
In the lumbar spine, motion preservation devices can be loosely grouped into anterior and posterior motion-preserving devices. anterior motion preservation devices
Anterior Motion Preservation Devices
These devices are intended to replicate the normal motion of the disc space.
Lumbar total disc replacement
Lumbar TDR devices vary widely in shape and composition. Following devices are in use
- Charite device (DePuy Spine)
- FlexiCore device (Stryker Spine)
- ProDisc-L device (Synthes Spine)
- NuVasive’s XL (Nuvasive)
Most of these devices require an invasive anterior approach. NuVasive’s XL is implanted indirectly through a far-lateral approach.
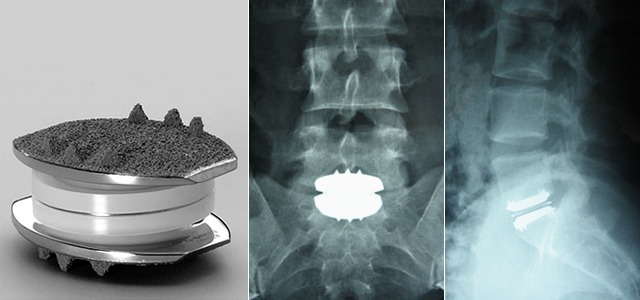
Image Credit: bnasurg
Complications associated with lumbar total disc replacement are hemorrhage, vascular injury, infection, peritoneal entry, vertebral fracture. Late complications of total disc replacement are subsidence of implant, implant migration, heterotopic ossification, adjacent-level degenerative disc disease
The clinical effectiveness has not been unequivocally determined.
Disc Nucleus Replacement
In partial disc replacement, the diseased nucleus pulposus is replaced or augmented with an injectable or preformed device. Annulus fibrosis is not replaced. The devices are in development
Posterior Motion Preservation Devices
These devices address pain originating from the posterior elements from the facet joints, ligaments, tendons, or muscles. Posterior motion preservation devices are also beneficial in spinal stenosis.
These devices can be put into three general categories:
Interspinous Process Spacers
These devices are designed to distract (open) the central canal and foramen[opening where the nerve roots come out of spinal canal]. These devices are designed to address pain and activity restrictions from spinal stenosis. Some of them may be used to treat degenerative disc disease.
The interspinous devices may be implanted as a day surgery procedure under sedation or under light anesthesia, thereby decreasing the risk of extensive open surgery in elderly.
Various interspinous spacer devices in use are X-STOP, Wallis interspinous device, and DIAM Spinal Stabilization System.
Posterior Dynamic Stabilization Devices
This acts as an internal brace for the spine. Posterior dynamic stabilization devices allow controlled motion in such as way as to achieve more normal movement of the spine. These devices are typically used to treat patients with spondylolisthesis and degenerative disc disease. As of now, these devices have been approved for use with fusion but not for independent use as dynamic stabilizers.
Facet Replacement or Total Element Replacement Devices
These are used in facet pain or lumbar spinal stenosis as in many patients, spinal stenosis is due to degeneration of the facet joints. Facet replacement devices replace the facet joints in the back of the spine to limit or control motion, and total element replacement devices replace all the elements in the back of the spine.
None of the devices have withstood the test of time, and most are still in various stages of investigation.
Kyphoplasty
Kyphoplasty is a minimally invasive treatment developed to treat vertebral compression fractures in the spine. Compression fractures cause back pain and make daily activities like walking and lifting difficult. The principle of the kyphoplasty is to strengthen the spine and possibly eliminate pain.
Read more about kyphoplasty
References
- J. Errico. Why a mechanical disc? Spine J, 4 (Suppl 6) (2004), pp. 151S–157S
- Yin, X. Yu, S. Zhou, et al. Is cervical disc arthroplasty superior to fusion for treatment of symptomatic cervical disc disease? A meta-analysis. Clin Orthop Relat Res, 471 (2013), pp. 1904–1919
- Wei, Y. Song, L. Sun, et al. Comparison of artificial total disc replacement versus fusion for lumbar degenerative disc disease: a meta-analysis of randomized controlled trials. Int Orthop, 37 (2013), pp. 1315–1325.
- Yajun, Z. Yue, H. Hiuxin. A meta-analysis of artificial total disc replacement versus fusion for lumbar degenerative disc disease. Eur Spine J, 19 (2010), pp. 1250–1261
- C. Wang, P.M. Arnold, J.T. Hermsmeyer, et al. Do lumbar motion preserving devices reduce the risk of adjacent segment pathology compared with fusion surgery? Spine, 37 (22S) (2012), pp. S133–S143
- E. Ziegler, J. Glenn, R.B. Delamarter. Five-year adjacent-level degenerative changes in patients with single-level disease treated using lumbar total disc replacement with ProDisc-L versus circumferential fusion. J Neurosurg Spine, 17 (6) (2012), pp. 504–511